Biocompatible Dental Material
10/05/2016 / By holisticdentistry

It is important to determine dental material biological compatibility (biocompatibility). There are three different levels of biocompatibility to consider: general, immunological, and bio-energetic.
Article by BioDentistryVizcarra.com
- General biocompatibility–On this most basic level, we have to look at how the material reacts generally with human tissue. In other words, how toxic the material is at a cellular level. Does it poison cells? This toxicity tends to be an inherent property of the material, and all people will react to it in a similar manner. Using a toxic material such as mercury or nickel would always be a mistake.
- Immunological biocompatibility–This level looks at materials from a standpoint of how an individual reacts to the material. The problem is analogous to what happens when someone with an allergy to mushrooms consumes an edible mushroom. The adverse reaction is due to an immunological and/or allergic type response based on teh patient’s biochemical makeup. A test like the Clifford Materials Reactivity Test (www.ccrlab.com) measures how antibodies in the blood react with the material (or, more accurately, its corrosion by-products).
This blood test indicates if the material is suitable form an indiviedual reactivity point of view. When evaluating a Cliffor test, a material that tests NS (not suitable) should not be used. On the other hand, materials that test S (suitable) should, ideally, be further tested. Kinesiologic testing for biocompatibility can also be performed to determine the body’s immunological response to substances. - Bio-energetic biocompatibility–For thousands of years Eastern medicine has studied the flow of energy through the human body and applied this to such healing arts as acupuncture. In more recent times, Dr. Reinhard Voll spent 40 years studying and documenting the relationship between teeth and organ systems. Using Electro dermal Screening (EDS) andApplied Kinesiology (AK or Muscle Testing), it can be determined how a material reacts with the body on an energetic level. If energetically incompatible materials are used, interferences are created on the meridians associated with the teeth being restored. according to Eastern medicine, each tooth correlates with and potentially influences different organ systems.
To select a restorative material of the highest levels of compatiblility, start with materials that exhibit low toxicity to cells, then eliminate the ones that elicit an immunological response, and then, to further narrow down the choice, select from the remaining materials using either EDS or AK. Using such a multi-step approach should give you the highest probability of selecting a non-reactive, compatible material. Of course, many patients will skip the testing and simply ask to have a material used that has been found to generally biocompatible. However, in cases where patients are experiencing multiple chemical sensitivity (MCS), various illnesses, or are highly concerned about achieving the highest level of biocompatibility, specific testing as described above is indicated.
Once the final restorative material has been selected, it is important ot understand that other materials are used in order to get a finished product. In the case of a metal restoration, this usually involves only the dental cement used to adhere the crown to the tooth (unless the tooth is so broken down that it requires a build-up, in which case all of the following steps also apply). If the restoration is a ceramic or composite, a number of steps are required to complete the bonding process.
Composite Fillings
These materials consist of tiny glass particles suspended in a resin (plastic) matrix. The size and amount of the glass particles give the composite filling materials different characteristics, such as strength and polishability. Composites are used in areas where the fillings are relatively small and there is plenty of tooth structure for support.
Composite fillings are less durable than amalgam if the filling is large, but comparable in durability if the filling is small to average size. Composite fillings in back teeth are significantly more difficult and time-consuming to place than amalgam fillings, therefore more expensive. Composite materials are most commonly placed directly into the tooth (like amalgam fillings), but can also be prefabricated and bonded into place indirectly (like a crown).
However, they are more natural-looking, require less tooth reduction to place, and are bonded in place for a better seal. Composites are not totally compatible either. Most are made of the petro-chemical bis-phenol, which some research indicates leaches out estrogen-like substances. Some composites are less biocompatible than others because of the amount of iron oxide, aluminum oxide, barium, and other materials in them.
Direct composites can cause hairline cracks in the tooth from the hardening process, whereas indirect composites do not because they are hardened in the lab. Porcelain is more natural-looking than composites, but because it is harder and more brittle, it causes a wearing away of everything it contacts with and can crack instead of flex from stress.
All porcelains contain aluminum oxide. The one exception is unshaded Dicor, which is weaker and not very natural-looking (over a period of 3-4 months, unshaded dicor will pick up the shade of the tooth under it). If metal is not used in a crown or bridge, it is significantly weaker and has an increased risk of breakage during normal function. Gold fillings, porcelain fillings, indirect composite fillings, and crowns require more tooth structure to be trimmed away than for amalgam and direct composites, and take two appointments rather than one. Most “gold” crowns placed today contain from 1% – 40% gold and have nickel in them, which is inappropriate for those with a compromised immune system. Special order higher content gold will obviously cost more due to the cost of gold.
Studies of gallium alloys have reported problems with corrosion, durability, tooth fracture, and tooth sensitivity. More research and development is needed, but for now, it is recommended for use in baby teeth only. Some experts consider all metals, even non-allergenic or non-toxic metals, to be disruptive and therefore believe they should never be used in the body. Since nearly all composites and porcelains contain iron and aluminum oxides, some experts limit their choice of materials to only a few.
Still others think the use of high quality metals like high content gold or titanium is acceptable, but only if one brand and formulation is used for the entire mouth. One must weigh biocompatibility against function and durability. Because of contractual language and statistics, use of titanium, high content gold, and composite for crowns, bridges, or fillings will probably result in lessened insurance benefits, even though the time, cost, and effort in doing them is the same or more as for standard gold alloy and porcelain materials. Cadmium is used for color stabilization, so cadmium-free materials may lose some color over time.
Filling Materials Amalgam
malgam is the most commonly used material for back teeth. It contains approximately 50% mercury and varying amounts of silver (30%), tin, zinc, and copper. It is the least costly and least time-consuming to place. It does not hold its shape over time, corrodes easily, and is expected to last 5-10 years. The controversy is that it contains mercury, a known neurotoxin (poison to the nervous system).
MERCURY
Number 80 on the “periodic table” of elements
Mercury or “quicksilver,” is a shiny liquid metal that is a widespread environmental contaminant. The levels of mercury in our bodies today are much higher because of its greater use in recent times. Mercury is employed daily by medical and dental practices in thermometers, drugs and amalgam for fillings. It is also present in fungicides and pesticides and in some cosmetics. Mercury from industrial waste has contaminated our fresh- and salt-waters and to the plants and fish therein.
An average body contains about 10-15 mg. of mercury. This comes daily from our food, air, and water. Mercury is not well absorbed through the intestinal tract, only about 5-10 percent. Inhaled mercury fumes go into the blood, since it is soluble and passes through the lungs. Some mercury is retained in body tissues, mainly in the kidneys. The kidneys store about 50 percent of the body mercury. The blood, bones, liver, spleen, brain, and fat tissue also hold mercury. This potentially toxic metal does get into the brain and nerve tissue, so central nervous system symptoms may develop. Mercury can also get into a growing fetus and into breast milk. Mercury is eliminated daily through the urine and feces. Urine levels would show whether the body is actively working to eliminate it.
Mercury has the following effects (simplified) on the body:
- disruption of the nervous system:
- damage to brain functions – degradation of learning abilities, personality changes, tremors, vision changes, deafness, muscle incoordination and memory loss ;
- DNA and chromosonal damage – chromosonal damage is known to cause mongolism;
- allergic reactions resulting in skin rashes, tiredness and headaches;
- disrupting reproductive functions such as sperm damage, birth defects and miscarriages
Toxicity Limits
The average overall daily intake is between 30-50 mcg. Acceptable levels are between 0.02 – 0.03 ppm depending on what was analysed. Readings above 0.05 ppm should be cause for concern.Mercury is a highly toxic heavy metal and concentration levels should be 0
Galloy
Galloy is a brand new material containing silver, tin, copper, indium, and gallium. It is meant to be mercury-free alternative to amalgam. Why is the American Dental Association developing & patenting this substitute for mercury amalgam if mercury amalgam is safe?
Direct Composite
A direct composite is a special plastic material that bonds to tooth structure, is tooth colored, is more easily repairable, and requires less tooth structure to be trimmed away than any other material. It is expected to last 5-7 years, although small to moderate size fillings may last longer. Research has shown that it reinforces the tooth and makes it stronger. Cost and time to perform is about 50-75% more than amalgam. Composites are a petrochemical derivative and, as such, are a possible problem for the environmentally sensitive.
Indirect Composite Inlay/Onlay
This type of restoration is used when ideal fit and durability is desired, which is seldom achieved with a direct composite filling. Cost is approximately 2-3 times that of an amalgam filling and takes two visits.
Porcelain Inlay/Onlay
Dental ceramics (sometimes referred to as dental porcelains) have come a long way! Until a few years ago, these materials were relatively weak (that’s why they required support from a metal substructure) and abrasive (causing wear on the opposing teeth). Today there are many different types of ceramic systems: Feldspathic, Leucite-reinforced, Polymer-reinforced, Zirconium-based–each with unique properties. From rebuilding broken teeth to replacing missing teeth (even in the back of the mouth), there is a ceramic to do the job. However, they are more difficult to use than conventionally cemented (non-bonded) crowns.
Picking the right ceramic for the job, proper tooth preparation, quality laboratory work, and meticulous cementation technique are all needed for a successful tooth restoration. It costs about the same as an indirect composite inlay/onlay and takes two visits. Most ceramic and resin-based materials contain metals in the form of oxides (such as aluminum) or even heavy metals (such as cobalt, barium or cadmium). These are usually added to give the amterials strength and improve their appearance. Sometimes they are added to make the restoration show up on x-rays. The number of materials that do not contain any of these products is very limited. However, the advantage of being oxide-free is lost when these are bonded to the tooth using an oxide-containing luting agent.
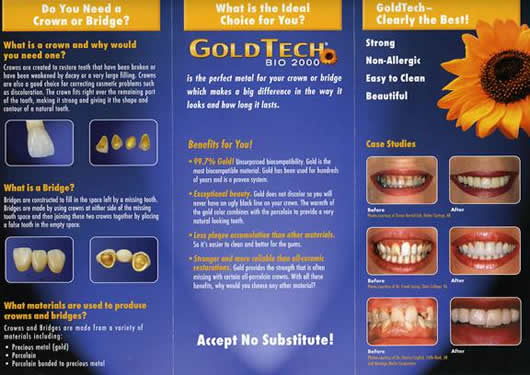
Gold Inlay/Onlay
Because of gold’s long history, it is the standard against which other materials are judged. This type of restoration is used when maximum strength is desired and appearance is not a factor. Gold is almost never used in its pure form; rather gold is used as an alloy with other metal elements. It costs approximately three to four times more than an amalgam and takes 2 visits. There are many formulations of gold, varying from 1% to 99%. The other metals are added in order to give the gold strength and the ability to bond to porcelain (in the case of porcelain veneer fused to cover a gold crown). The most commonly added metals are palladium, silver, copper, and platinum.
Biocompatible High Noble Gold 99.7 % Crowns fused to Porcelain.
Free Palladium ,Free Silver, Free Cooper, Free Platinum
Holistic Dentistry
Argen understands your need for holistic, hypo-allergenic alloys and offers a variety of alloys to meet your needs, including Argedent Bio Yellow PF.
The composition and amount of each metal in the alloy determines whether it is classified as a “high noble,” “noble,” or “base” metal. “Noble” metals are defined as gold, platinum and palladium. The most expensive gold alloys are “high noble” and they are defined as hiving at least 60% noble metals and at least 40% gold. An alloy can still be called “noble” if it has at least 25% noble metal content. The cheapest materials fail even that test and are called “base” alloys–they have less than 25% noble metals. It is especially important for patients with metal sensitivities to avoid the base alloys since these usually contain toxic metals such as nickel and chromium;. But even the high noble materials can be incompatible for patients and even toxic; palladium, for example, is toxic.
Titanium Inlay / Onlay
Titanium is used when a gold alloy is not biocompatible; otherwise, the benefits, cost, and time to perform are the same as for a gold alloy, even though it is not a precious metal. It takes two visits.
Palladium Dental Alloy
Palladium ( Pd ) is a silver-white ductile and malleable metal that belongs to the platinum groups it is atomic weight is 106: it is melting point 1557 degrees Celsius. Palladium is found in the platinum mines of Russia, Canada, Columbia, and is used in electrical contacts as a catalyst and in Gold, Silver and Cooper Alloy. The use of Palladium in Dentistry dates back to 1986, and health insurances supported its use for what seen to be economic reasons .Only German Holist Medical doctors refer to Palladium as the “ Fool’s Gold” of dentistry because it may be mire dangerous than Mercury. The amount of Palladium used in the making on Dental Crowns varies considerable. The more durable Gold and Silver fillings or Crowns may contain up to 78.5 % of this Heavy Metal. Like Mercury Palladium is bio methylated in the digestion tract and once synthesized these methylated metals are invariable more toxic than their inorganic substrates.
This toxicity is thought to be due to the non polar nature of many organometallic compounds which allows them to diffuse rapidly into and though cell membranes. Dr. Elef Karkalis M.D. of Oppenheim Germany , believes that methylated Palladium is more than Methylated mercury , which is known to cause severe Neurological disorders, including insanity . MethylMercury interacts with phospholipids, causing neurotoxicity, MethylMercury poisoning has affected people in Japan, Guatemala, Russian and Iraq, and the industrial dumping of mercury into Lake St. Clair touched of hunt for Mercury –contaminated fish that invariable cuased human illness.
Since Palladium is easily Methyltated , it is considered to be equally or possible more dangerous to the health than Mercury , and the extensive use of Palladium Dental Crowns may indeed be a “Fool’s choice “. By exchanging “‘GOLD ‘ “ with other metal such as Nickel, Chrome , Cobalt , Copper. This replacement therapy may be more expensive health wise , Dr. Karkalis is most concerned about the enzyme blocking function of Palladium . Dr. Karkalis specifies early symptoms of Palladium over exposure as” Chronic fatigue, Allergies, Headaches, Lymph node swelling, Immune weakness. Specifies symptoms of advanced toxicity as : Bronchitis, Muscle and join pain, Memory loss, Digestive and nervous disorders, Weight loss, Chronic sinusitis and tinnitus, excessive sweating, Neuralgia, Facial paralysis, Sleeping disorders, Muscle weakness.
According to German sources Gold Dental Crowns contain a maximum of 18 % Gold ( Au ) and aside from containing Palladium , other metal compounds such as Silver, Cooper, Platinum , may be used. Metallic silver ( Ag ) has been reported as solid state carcinogen while soluble compounds of silver increased DNA miscopying in vitro. The deterioration of metallic compounds such Mercury, Silver, Palladium, Amalgams increases the oral intake of potentially TOXIC METAL , yet unlike Mercury Amalgams which are know to slowly induce symptoms of Mercury Toxicity , Palladium Dental Alloy Crowns seem the induce toxicity problems much sooner.
Palladium used in Dental Alloy Gold Crowns since 1989. May more toxic than Mercury Dental Fillings . Some “Gold Alloy “ dental fillings like crowns, fixed bridge, inlay, onlay , Meryland bridge , contain up to 78.5 % of Palladium ( Pd ) . Hair, urine and blood analysis detects long – term chronic exposure ad sub-clinical toxicity. High blood and urine levels are considered signs of a severe chronic or acute toxicity.
Trace Mineral International Inc.
Dental Material Philosophies Conventional
Except in rare situations, currently used dental materials are safe in the mouth. The important criteria are how durable, natural looking, inexpensive, and practical they are for the dentist and dental laboratory to use. Concerns therefore are economics and aesthetics. Because some people have a sensitivity to certain substances, the choice of dental materials may have to be limited. A special blood test may be used to determine sensitivity to corrosion by-products of components.
Toxicity
Some dental materials contain toxic substances that, depending on exposure and other factors, may impact total toxic body load. Non-toxic alternatives should be used to decrease exposure to and accumulation of scientifically confirmed environmental toxins. Some dental treatment and materials can be disruptive to the normal flow of energy through the acupuncture meridians. Eastern philosophy believes chronic disruption of energy flow causes dysfunction and resultant health problems on the associated meridian; therefore, the choice of dental materials and treatments is limited.
Electrogalvanism
Dissimilar metals in the mouth, including different formulations of the “same” metal, create microamps of current which could cause oral pain, corrosion of the metal (black mercury amalgam fillings), dry mouth, metallic taste, erythema (red & swollen gums), and possible dysfunction of other organ systems, endocrine glands, etc. on that meridian.
Crown and Bridge Materials
Gold Alloy
A gold alloy is used when maximum strength is desired and appearance is not a factor. There are many formulations of gold, varying from 1% to 99%.
Titanium
Titanium is used when maximum strength is desired, appearance is not a factor, and a gold alloy is not biocompatible. There are different purities of titanium, with grade-1 being the purest. This is used in joint replacement, dental implants, and bone pins. Cost is the same as for gold alloy.
Non-Precious Alloy Nickel heavy metal poisoning
Non-precious alloys are used when maximum strength is desired, appearance is not a factor, but cost is most important. Since it does not contain any gold, cost is less. There are two basic formulations, one that contains nickel and one that is nickel-free. The controversial issue is that nickel, beryllium, cobalt, chromium, and palladium may cause immune problems and/or toxicity.
Toxicokinetics
- Absorption
Health hazards associated with exposure to nickel in the occupational environment have resulted primarily from inhalation. However, in addition to this main exposure route, workers are also indirectly exposed to nickel by ingestion of drinking-water and food, and through skin contact. Tobacco smoking is also an important source of nickel exposure.
The relative amounts of nickel absorbed by an organism are determined, not only by the quantities inhaled, ingested, or administrated, but also by the physical and chemical characteristics of the nickel compound. Solubility is an important factor in all routes of absorption, following the general relationships nickel carbonyl absorption> soluble nickel compounds absorption> insoluble nickel compounds absorption.
Nickel carbonyl is the most rapidly and completely absorbed nickel compound (in both animals and human beings).-inhalation exposure: respiratory absorption of nickel is normally the principal route for its entry into the human body under conditions of occupational exposure. It usually involves the inhalation of one of the following substances: dust of relatively insoluble nickel compounds (particulate nickel), aerosol derived from nickel solutions or gaseous forms containing nickel (e.g. nickel carbonyl).Particulate nickel: respiratory absorption of nickel compounds in particulate form is influenced by three processes in the lung: deposition, mucociliary clearance, and alveolar clearance. Moreover, the deposition pattern in the respiratory tract is related to particle size, which determines the degree to which particles are affected by inertial impaction, sedimentation, and diffusion. In humans, about 20-35% of the inhaled less-soluble nickel (nickel oxide, nickel subsulfide) that is retained in the lungs is absorbed into the blood. The remainder is either swallowed, expectorated, or remains in the respiratory tract.Soluble compounds are more readily absorbed from the respiratory tract as indicated by the higher concentrations of urinary nickel found in workers exposed to e.g. nickel chloride or nickel sulfate compared to those exposed to less-soluble nickel compounds.Nickel carbonyl: in the toxicology of nickel, a special position is occupied by nickel carbonyl, a volatile, liquid, liposoluble compound. After nickel carbonyl inhalation, removal of nickel deposited in the lung is the most rapid, compared with the clearance of all other compounds, indicating an extensive absorption and clearance. Nickel carbonyl is the only one of the nickel compounds to cause acute symptoms of poisoning, when inhaled.
-oral exposure: absorption of nickel from the gastrointestinal tract occurs after ingestion of food, beverages, or drinking-water. A significant quantity of inhaled material is also swallowed following mucociliary clearance from the respiratory tract. Poor personal hygiene and some work practices can contribute to gastrointestinal exposure.
The rate of nickel absorption from the gastrointestinal tract is dependent on its chemical form and may be influenced by some factors as composition of the diet, or interactions with other elements.
Soluble nickel compounds (i.e. nickel sulfate) are better absorbed than relatively insoluble ones after ingestion(and/or inhalation). However the contribution of the poorly soluble compounds to the total nickel absorption may be more significant after oral than after inhalation exposure since they are more soluble in the acidic gastric fluids.Factors influencing gastrointestinal absorption are: composition of the diet
Gastrointestinal absorption of nickel is variable and depends on the composition of the diet. In human volunteers who ingested nickel sulfate in the drinking-water or food, at doses of between 12 and 50 µg/kg body weight (one treatment), absorption of nickel averaged 27 (± 17%) of the dose from water compared with 0.7 (± 0.4%) of the same dose from food.
It was also reported that nickel absorption may be suppressed by binding or chelating substances, competitive inhibitors, or redox reagents; on the other hand, absorption is often enhanced by substances that increase pH, solubility, or oxidation, or by chelating agents that are actively absorbed. Such compounds include: ascorbic acid, citric acid, pectins (from orange juice), which affect trace mineral absorption; tannins (in tea and coffee), which inhibit absorption of iron and zinc; ascorbic acid which suppresses nickel absorption; and complexing agents, such as EDTA, wich depress plasma-nickel levels.nickel/iron interaction
Some authors have suggested that the iron nutritional status may influence nickel absorption-dermal exposure: cutaneous absorption of nickel is quantitatively minor, but it may be important in the pathogenesis of contact dermatitis caused by nickel hypersensitivity.
Because of the ubiquity of nickel-containing objects in industrialised societies, nickel-sensitive patients frequently face considerable problems at the workplace in a
wide range of jobs. - Distribution
There are few data on human tissue concentrations of nickel. The levels of nickel in biological fluids, hair, and some other materials increase remarkably in persons with increased occupational or environmental exposure and decline rapidly when exposure is reduced or stopped. Thus, measurements of nickel, particularly in the urine, serum or hair, may serve as indices of exposure. The normal ranges of nickel concentrations in body fluids or tissues (serum, blood, lung, kidney) are not significantly influenced by age, sex, or pregnancy.
Metabolism
Nickel is taken up by the lungs and via the peroral route, but ionizable nickel compounds do not pass through the intact skin. Once it has entered into the circulation, 75% of plasma nickel is carried by the circulating proteins, e.g. albumin, a2-macroglubulin and nickeloplasmin. As Ni2+ ions have no specific uptake mechanism, they probably enter the cells by way of Ca2+/Mg2+ channels. Water soluble nickel salts are generally not carcinogenic, probably because they cannot enter cells. Water insoluble nickel compounds can produce their carcinogenic response because they are actively phagocytized into the cells. Because of the 24h half-life of Ni2+ leads to an accumulation of nickel in the body during the workweek, biological monitoring for occupational nickel exposure is most efficient at the end of weekly workshift. One should, however, keep in mind that this statement applies only to soluble nickel compounds. Phagocytized nickel compounds may be released slowly, causing high urinary nickel concentrations even weeks or months after exposure has ceased (Savolainen, 1996; Patriarca et al, 1997).
References
- Patriarca, Lyon and Fell. Nickel metabolism in humans investigated with an oral stable isotope; American journal of clinical nutrition, 1997, 66, 616-21.
- Savolainen. Biochemical and clinical aspects of nickel toxicity; Reviews on environmental health, 1996, 11, 167-173.
- Snow. Metal carcinogenesis: mechanistic implications; Pharmacology and therapy, 1992, 53, 31-65.
-
Elimination
The elimination routes for nickel in human beings (and animals) depend, in part, on the chemical form of the compound and the mode of intake.
Urinary excretion is the major route for the elimination of absorbed nickel. Fecal excretion primarily reflects the nickel that is unabsorbed from the diet and passes through the gut.
Other routes of elimination are of minor importance. All body secretions appear to have the ability to excrete nickel; it has been found in saliva, sweat, tears, and milk.
Biliary excretion is minimal in animals, but may be significant in human beings. Hair is also an excretory tissue for nickel.
In nickel workers, an increase in urinary excretion was found from the beginning to the end of the shift, indicating absorption of a fraction that was rapidly eliminated.
An increase in urinary excretion was also found as the workweek progressed, indicating a fraction that was excreted more slowly.
For persons exposed to soluble nickel compounds, nickel concentrations in body fluids are generally elevated after exposure but the relationship with the intensity of exposure is relatively poor. Among electrolysis and refinery workers exposed to soluble nickel compounds (nickel sulfate aerosols), nickel concentrations in the urine were 5.2-22.6 µL for those exposed to concentrations of 0.11-0.31 mg Ni/m3, and 3.2-18 µg/L for those exposed to 0.08-0.2 mg Ni/m3. Nickel is also excreted in the faeces of the workers, but this probably results from mucociliary clearance of nickel from the respiratory system to the gastrointestinal tract or from oral exposure via contaminated surfaces.
Increased concentrations of nickel in urine may also be observed in workers exposed to less-soluble nickel compounds and are indicative of significant nickel
absorption
2. Target organs
The skin and the respiratory tract are the principal target organs upon occupational exposure.
Skin: nickel and nickel compounds have a strong sensitising potential on the skin, which is manifested by irritation, eczema and allergic contact dermatitis. Oral
intake of low doses of nickel may provoke allergic dermatitis in sensitised individuals.
Respiratory tract: Beside the carcinogenic effects on lung and nasal cavities associated with an exposure to nickel, other respiratory effects have been described:
-epithelial dysplasia (possibly representing precancerous lesions)
-pathological changes of the nasopharynx (nasal septal erosions, perforations, ulcerations)
-hyperplastic/polypoid rhinitis
-hypo-osmia and anosmia
-chronic sinusitis/bronchitis
-decreased pulmonary residual capacity, increased respiratory frequency
-pneumoconiosis
-fibrotic changes
-allergic asthma.
A single case of death from acute respiratory distress syndrome has been reported in a adult following exposure of small size nickel metal particles (<1.4 µm)
Renal effects: biochemical indications of nephrotoxicity in nickel workers have been observed: a significant association was found between nickelemia and increased urinary markers reflecting tubular dysfunction (i.e. b2 – microglobulin).
Nickel carbonyl is the most acutely toxic nickel compound. Symptoms following nickel carbonyl intoxication occur in two stages, separated by an almost symptom-free interval which usually lasts for several hours. Initially, the major symptoms are nausea, headache, vertigo, upper airway irritation and substernal pain, followed by interstitial pneumonitis with dyspnoea and cyanosis. Prostration, pulmonary oedema, kidney toxicity, adrenal insufficiency and death may occur in severe cases.
Frequent clinical findings include fever with leukocytosis, electrocardiographic abnormalities suggestive of myocarditis and chest X-ray changes.
Hyperglycaemia has also been reported.
3. Teratogenicity:
Only few data are available on the reproduction and developmental effects of nickel in human beings.
-inhalation exposure: an increase in spontaneous abortions and in structural malformations was reported among women who worked in a nickel hydrometallurgy refining plant in the arctic region of Russia. However, the investigators noted that the nickel-exposed women manually lifted heavy nickel anodes and they may have experienced heat stress.
-oral exposure-dermal exposure: no studies were located regarding reproductive or developmental effects in humans.
-transplacental transfer: nickel has been shown to cross the human placenta. Measurable concentrations have been found in various fetal tissues (e.g. liver, kidney, brain, heart, lung, skeletal muscle, and bone) and in the umbilical cord serum, where the average concentration from 12 newborn babies was 3±1.2 µg/L and was identical with that in the mother’s serum, immediately after delivery.
Placental transfer is influenced by gestational age and the availability of nickel in the maternal blood.
The passage of nickel across the human placenta barrier is of relevance because of the presence of female workers in industry. Appreciable amounts of nickel have been found in breast milk.
4. Genotoxicity:
The DNA alterations responsible for the carcinogenic process are generally thought to occur in genes. In view of the positive response obtained in the SCE test, additional experiments should be performed to test the ability of nickel compounds , such as nickel carbonyl, displaying marked carcinogenic and teratogenic properties, to induce gene mutations in mammalian cells
5. Carcinogenicity:
Some nickel species are carcinogenic.
Epidemiological studies have demonstrated a strong association between lung and nasal cancers and exposure by inhalation to nickel compounds in nickel refineries, primarily (in) the early stage of nickel refining. The excess risk of cancer seems to be mainly associated with exposure to less-soluble compounds (i.e. nickel sub sulfide, nickel oxide). However, observations conducted in the electrolysis and in the preparation of soluble salts departments suggest that there might also be an association between soluble nickel and respiratory tract cancers.
Excesses of various cancers other than the lung and nasal cavities, such as renal, gastric, or prostatic cancer, have occasionally been reported in nickel workers, but none has been found consistently
In recent years there has been a dramatic increase in the use of nonprecious alloy and porcelain crowns in clinical dentistry. The alloy in these restorations frequently contains a high percentage (greater than 70%) of nickel. Most cases of metal hypersensitivity are related to nickel, and clinical manifestations of the hypersensitivity are the result of a cellular (T lymphocyte) immune response. In this report, we review the cases of two women who demonstrated significant loss of alveolar bone about nickel-rich nonprecious alloy and porcelain crowns. The loss of alveolar bone occurred within 18 months after placement of the restorations. Both individuals displayed a positive patch test to a nickel preparation. These findings suggest that a Type IV hypersensitivity reaction may have accounted for the rapid loss of alveolar bone. Though the majority of individuals treated with nonprecious alloy and porcelain crowns apparently tolerate these restorations quite well, greater care is urged in case selection.
We report a patient with documented IgA nephropathy in whom microscopic hematuria, proteinuria, and hypertension first occurred after placement of nickel alloy base dental crowns. Progressive proteinuria culminating in nephrotic-range proteinuria occurred parallel to increased nickel placement and dramatically resolved following nickel alloy removal. That immunologic alterations occur as a result of nickel exposure has already been suggested by the common occurrence of nickel contact dermatitis, often exacerbated by intraoral nickel placement, increased carcinogenesis in nickel refinery workers, and animal models of nickel-associated carcinogenesis. Our patient may represent an example of nickel-induced sensitization and associated IgA glomerulopathy. Further study of patients with immune-mediated glomerulopathy with attention to dental nickel exposure appears indicated.
As the cost of providing dental care escalates, there has been a substantial increase in the substitution of nonprecious alloys for gold and precious metals found in porcelain-fused-to-metal crowns. These alloys are frequently 69% to 81% nickel. Nickel hypersensitivity is quite common in the general population and periodontal responses have been associated with nickel-containing crowns in nickel-sensitive individuals. In this article, the authors report the case of a nickel-sensitive patient who demonstrated loss of alveolar bone after the placement of crowns with a high nickel content. Nonprecious alloy crowns can not be well tolerated in most cases, a history of metal sensitivity should be evaluated.
Clinical use of the new base-metal alloys in restorative dentistry involves a risk for both dentist and patient. It is the responsibility of the dentist to determine if a patient is allergic to nickel prior to treatment with a restoration containing a nickel alloy. A patch test is recommended for nickel sensitivity in every patient when such a restoration is planned. In addition, the dentist should include in the work authorization order to the dental laboratory the type of alloy he wants for a particular patient. The dentist should be prepared to check for the presence of nickel in a casting suspected of containing it using the dimethylglyoxime test. The evaluation record for nickel sensitivity should include the patient’s name, age, history of allergies, medication, name of drug, dosage, and reaction. The record should be kept in the patient’s chart.
Cancer of the lungs, nasal mucosa and less frequently , of the larynx account for the most serious consequences of usually long term occupational exposure to Nickel.
The first case of the respiratory tract cancer were noted in 1932, in Wales, nickel refinery. It has been established that nickel sulfide and nickel oxide are the most carcinogenic agents of all the nickel compounds encountered. Workers engaged in the nickel manufacture and refinery appear to run the highest risk of exposure to nickel compounds. It emphasized the likelihood of nickel –exposed workers to develop ling cancer as significantly higher among smokers than nonsmokers, particularly asbestos workers. Nickel like Beryllium compounds , has been capable of damaging DNA and impairing its synthesis in certain systems. Available data is considered inadequate for classifying it as convincingly genotoxic. Apart from being carcinogenic, Nickel is also a commonly know producer of local inflammatory reaction of the skin, chiefly due to sensitization. In workers of a Nickel refinery, and even in children residing in the vicinity of the plant, there were found significantly elevated levels of some serum proteins classed among so called acute reactants. Nickel and nickel compounds are know to play a significant role in the etiology of eczematous processes on the human skin. It is estimated that about 5%-13% of all cases of eczema are caused by contact with Nickel or nickel compound s. Nickel dermatitis, sometimes referred to as nickel itch, may both is occupationally ad non- occupationally exposed individual especially in women as a result of direct contact with stainless steel and nickel – plated articles, such as coins, cooking utensils, watches, costume jewelry , etc. Leaking of Nickel from these metals that assists in skin absorption resulting in allergic reaction and dermatitis, may be enhanced by sweat and cosmetics. For the action of adverse biological effects of Beryllium and Nickel of primary importance is their solubility in water, serum and from the oral view point , in the saliva. So far, no cases of malignant tumor at sites of dental prostheses have been reported, but local sarcoma have developed in humans and domestic animals at sites of metallic prostheses made of Nickel. Chromium and Cobalt alloys. Nevertheless , among these netals Nickel is the most potent Carcinogen.
The above mentioned adverse health effects, namely immunotoxicity and carcinogenicity of both metals , fully justify cautiousness in their application in materials designed for the production of Dental Partial Prosthetics. They represent potential health risk aspects of the usage of nickel alloys for these purposes and because of the high sensitization potential of Beryllium to exclude Beryllium al together from the preparation of metal alloys for prosthetic and other material, namely from cements used to fix crowns and bridge, Future increased application of these materials, especially Beryllium alloys in Oral practice would represent undesirable and simple avoidable sources of potential health risk for the general population. Instead of these Beryllium alloys are alternatives of classical materials, besides alloys which is from the point of view of biological aggressively safer material accordin to the experience of surgical practice.
HAZARD ASSESSMENT
Nickel dusts and several water-insoluble nickel compounds, especially the oxide and subsulfide, are carcinogenic in animals after inhalation or parenteral administration (Ottolenghi et al., 1975; IARC, 1976; Sunderman, 1984; Horie et al., 1985). Accordingly, increased incidences of pulmonary and nasal cancers in nickel refinery workers have been attributed to the inhalation of nickel compounds (Sunderman, 1977). However, there is no indication from the available evidence that ingestednickel is carcinogenic in humans or in laboratory animals.
While nickel chloride was not mutagenic in several bacterial assay systems (EPA, 1985), nickel did produce chromosomal aberrations in cultured mammalian cells, and sister chromatid exchanges in both cultured mammalian cells and human lymphocytes (Miyaki et al., 1979; Ciccarelli et al., 1981; Saxholm et al., 1981; Newman et al., 1982; Robison and Costa, 1982). The relevance of these findings to the toxicity of nickel compounds in vivo remains to be determined.
The World Health Organization (WHO) has not yet suggested a tolerable intake level for nickel (Private Communication 7, June 26, 1990).
The EPA’s current oral Reference Dose (RfD) for soluble inorganic nickel is 20 ![]() g/kg/day. This value was calculated by applying an uncertainty factor of 100 and a modifying factor of 3 to a no observed adverse effect level (NOAEL) of 5 mg/kg/day obtained in a 2-year rat feeding study (Ambrose et al., 1976). Ambrose et al. (1976) studied clinical pathology, ophthalmology, serum biochemistry, body, and organ weight changes, and performed histopathologic evaluations of selected organs (heart, kidney, liver). The EPA’s modifying factor of 3 was applied to the NOAEL, in addition to the uncertainty factor of 100, to compensate for the statistical and other inadequacies in the available reproductive studies (RTI, 1987; Ambrose et al., 1976). A tolerable daily intake of 1,200
g/kg/day. This value was calculated by applying an uncertainty factor of 100 and a modifying factor of 3 to a no observed adverse effect level (NOAEL) of 5 mg/kg/day obtained in a 2-year rat feeding study (Ambrose et al., 1976). Ambrose et al. (1976) studied clinical pathology, ophthalmology, serum biochemistry, body, and organ weight changes, and performed histopathologic evaluations of selected organs (heart, kidney, liver). The EPA’s modifying factor of 3 was applied to the NOAEL, in addition to the uncertainty factor of 100, to compensate for the statistical and other inadequacies in the available reproductive studies (RTI, 1987; Ambrose et al., 1976). A tolerable daily intake of 1,200 ![]() g Ni/day for a 60-kg individual can be suggested on the basis of the EPA’s current RfD (20
g Ni/day for a 60-kg individual can be suggested on the basis of the EPA’s current RfD (20 ![]() g/kg/day x 60 kg = 1,200
g/kg/day x 60 kg = 1,200 ![]() g/day).
g/day).
An additional concern not yet addressed in the EPA’s estimate for the RfD for nickel {and the proposed maximum contaminant level (MCL) corresponding to this RfD} is that acute oral nickel ingestion, added to a normal diet, may produce adverse reactions in persons with nickel hypersensitivity. Hypersensitivity to nickel is a common cause of allergic dermatitis in people (Sunderman, 1988). Sensitization due to cutaneous contact with nickel or nickel compounds is observed in 5-13% of various populations exhibiting eczema (NAS, 1975). Furthermore, positive dermal patch tests for nickel were observed in 7-11% of women and 0.2-2% of men from the general population. Nickel hypersensitivity has been attributed to direct cutaneous exposure to stainless steel utensils and other nickel-containing or nickel-plated objects such as coins, watches, jewelry and clothing fasteners (Norseth, 1986; Sunderman, 1988). Nickel hypersensitivity may also cause pulmonary asthma, conjunctivitis, and inflammatory reactions around nickel-containing implants and prostheses (Arvidsson and Bogg, 1959; Sunderman, 1988).
A double-blind study conducted by Gawkroder et al. (1986) showed that a single 5,600 ![]() g oral dose of nickel worsened the skin conditions in all 6 of the subjects challenged with this dose, confirming the results of Christensen and Moller (1975). Gawkroder et al. (1986) suggest that oral nickel intake as high as 5,600
g oral dose of nickel worsened the skin conditions in all 6 of the subjects challenged with this dose, confirming the results of Christensen and Moller (1975). Gawkroder et al. (1986) suggest that oral nickel intake as high as 5,600 ![]() g can aggravate the eczematous skin conditions in nickel-hypersensitive persons.
g can aggravate the eczematous skin conditions in nickel-hypersensitive persons.
Kaaber et al. (1978) reported that 17 of 28 patients with chronic nickel dermatitis exhibited aggravated eczematous reactions after ingesting a single 2,500 ![]() g dose of nickel as nickel sulfate in a tablet. According to Kaaber and his coauthors, the protocol in this study included a “blind challenge with tablets of identical appearance … carried out by giving each patient a placebo tablet and three days later a tablet containing 2,500
g dose of nickel as nickel sulfate in a tablet. According to Kaaber and his coauthors, the protocol in this study included a “blind challenge with tablets of identical appearance … carried out by giving each patient a placebo tablet and three days later a tablet containing 2,500 ![]() g nickel sulfate.” The patients were photographed 3 days after the ingestion of each tablet, and the photographs were then evaluated “blindly”. Thus, this study, like that of Gawkroder et al. (1986), appears to be a double-blind study.
g nickel sulfate.” The patients were photographed 3 days after the ingestion of each tablet, and the photographs were then evaluated “blindly”. Thus, this study, like that of Gawkroder et al. (1986), appears to be a double-blind study.
Veien et al. (1983) reported that three patients with positive patch tests to nickel developed flares at the patch test site after a single oral dose of 2,500 ![]() g nickel. Accordingly, Kaaber et al. (1979) reported that eczema was aggravated in 11 nickel-hypersensitive patients ingesting a single dose of 2,500
g nickel. Accordingly, Kaaber et al. (1979) reported that eczema was aggravated in 11 nickel-hypersensitive patients ingesting a single dose of 2,500 ![]() g nickel. In two of these patients, with particularly long-standing (10-17 years) hand dermatitis, a single dose of 600
g nickel. In two of these patients, with particularly long-standing (10-17 years) hand dermatitis, a single dose of 600 ![]() g or 1,250
g or 1,250 ![]() g nickel sulfate was sufficient to produce dizziness and nausea as well as exacerbated skin conditions.
g nickel sulfate was sufficient to produce dizziness and nausea as well as exacerbated skin conditions.
Furthermore, Cronin et al. (1980) reported that ingestion of a single dose of 600 ![]() g or 2,500
g or 2,500 ![]() g nickel, administered after an overnight fast, produced a dose-dependent enhancement of hand eczema in female patients known to have been sensitized from nickel contact. Of the five women receiving 600
g nickel, administered after an overnight fast, produced a dose-dependent enhancement of hand eczema in female patients known to have been sensitized from nickel contact. Of the five women receiving 600 ![]() g nickel, one developed erythema, two presented with worsening of hand eczema, and 3 exhibited flare of the patch test site. Gawkroder et al. (1986) state in their introduction that the reports of Cronin et al. (1980), Kaaber et al., 1979, Veien et al., 1983, and other researchers were not confirmed by two double-blind studies (Jordan and King, 1979; Burrows et al., 1981). However, Gawkroder and his colleagues also mentioned that a subject in one of these double-blind studies (Jordan and King, 1979) consistently reacted to a 500
g nickel, one developed erythema, two presented with worsening of hand eczema, and 3 exhibited flare of the patch test site. Gawkroder et al. (1986) state in their introduction that the reports of Cronin et al. (1980), Kaaber et al., 1979, Veien et al., 1983, and other researchers were not confirmed by two double-blind studies (Jordan and King, 1979; Burrows et al., 1981). However, Gawkroder and his colleagues also mentioned that a subject in one of these double-blind studies (Jordan and King, 1979) consistently reacted to a 500 ![]() g oral nickel challenge.
g oral nickel challenge.
In summary, one double-blind study showed that a single 2,500 ![]() g dose of orally administered nickel was sufficient to aggravate the chronic nickel dermatitis in 17 of the 28 patients tested. Other, less reliable studies suggest that as little as 600
g dose of orally administered nickel was sufficient to aggravate the chronic nickel dermatitis in 17 of the 28 patients tested. Other, less reliable studies suggest that as little as 600 ![]() g or 1,250
g or 1,250 ![]() g of ingested nickel may exacerbate the skin conditions in patients with long-standing (10-17 years) nickel hypersensitivity. In one double-blind study (Jordan and King, 1979), one of the 10 nickel hypersensitive patients tested consistently reacted to a 500
g of ingested nickel may exacerbate the skin conditions in patients with long-standing (10-17 years) nickel hypersensitivity. In one double-blind study (Jordan and King, 1979), one of the 10 nickel hypersensitive patients tested consistently reacted to a 500 ![]() g oral nickel challenge. Thus, oral nickel exposure of as little as 500
g oral nickel challenge. Thus, oral nickel exposure of as little as 500 ![]() g may produce adverse reactions in some nickel hypersensitive persons. A tolerable intake for nickel of 50
g may produce adverse reactions in some nickel hypersensitive persons. A tolerable intake for nickel of 50 ![]() g/day can be derived by applying an uncertainty factor of 10 to the lowest observed effect level for dermatitis in hypersensitive individuals.
g/day can be derived by applying an uncertainty factor of 10 to the lowest observed effect level for dermatitis in hypersensitive individuals.
Porcelain
Porcelain is used when appearance and wear resistance is the most important factor. It is much more fragile than metal and may break easily. Porcelain alone is not normally recommended for bridges.
Indirect Composite
Indirect composites are used when appearance is an important factor but when the risk of porcelain fractures and wearing down the other teeth is to be avoided. These are not quite as wear-resistant or esthetic as porcelain but very acceptable for normal situations.
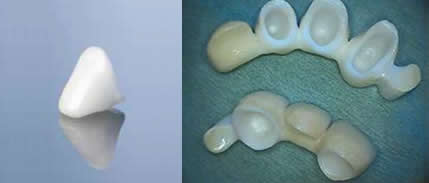
Biocompatible Zirconium Oxide All Ceramic Crowns
All Ceramic Restorations
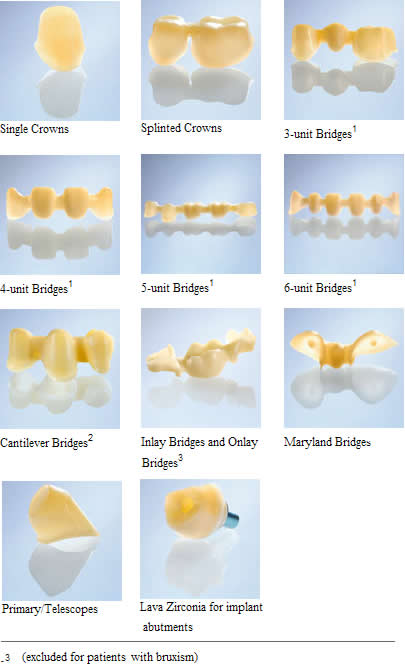
One of the most difficult areas in dentistry today is the restoration of dental structures with biocompatible materials that are strong enough to withstand the forces of chewing (500-1000lbs pressure on molar teeth). Recent technology from Germany now offers a material that has overcome most of the pitfalls of present day products. Patients now have a choice of a material that is esthetic, strong, pure, biocompatible and capable of being used for single and long span dental bridgework. That material is called Zirconium oxide.
Zirconium oxide has the following superior characteristics that make it the most ideal material available:
* Excellent biological compatibility: absolutely bio-inert.
* Outstanding physical and mechanical qualities:
* Hardness (Vickers) 1200 HV
* Compressive Strength 2000 MPa
* Bending Strength 1000 MPa
* Modulus of Elasticity 210 GPa
* Tensile Strength 7 Mpavm
* Wear characteristics (Ring on disc) <0.002 mm 3/h
* Absolute corrosion resistance: Ringer’s solution 370C <0.01mg/ m2x24h
* Very small particle size: <0.6ym
* No glass phase for particle binding
* Extremely high density
* Porosity: 0%
* Purity (Zr/Hf/Y): 99.9%
* Translucence of the framework material makes excellent cosmetic results possible
* Equivalent fit to precision gold castings: edge opening 20-50 ym. Precludes the need to use adhesive cements.
* Zirconium oxide is manufactured and optimized industrially so that the material qualities remain unchanged through the complete pro duction chain.
* Optimal material for crowns: tasteless, radiopaque, no pulp irrita tion because there is no need to use adhesive cements and minimal invasive preparation by dentist.
Zirconium oxide forms the core of each crown and provides the cross-link that bridges the gap of missing teeth. The precision fit of the Zirconium core is derived from computer guided Swiss lathes that cut the form out of a solid Zirconium oxide block. The cutting instructions are obtained from a laser beam that reads 120 points per millimeter from the anatomy of a model of the prepared teeth. Once formed, new synthetic porcelain (99.9% pure) is baked on to the Zirconium core and then shaped like a tooth. Because of the extreme accuracy of the crown fit, the crowns can be cemented with biocompatible dental luting material. This avoids the use of an invasive procedure of etching the tooth with acid and injuring the pulp or nerve of the tooth. This latter procedure often times results in the pulp dying and necessitating root canal therapy.
Advantages of ET zirconium high performance ceramic compared with other full ceramics.
Zirconium oxide ceramic primarily stands out due to its high crack resistance. Crack resistance is the resistance with which the material counteracts the spreading of cracks. If a material is stressed, it usually comes to excessively high tension within a defect area (pores, surface deficiencies, cavities) or it cracks. While with metals under high tension in the area of cracks, plastic deformation appears and the top of the tension can be reduced by rounding the cracks; in ceramics due to missing plastic deformation possibility the cracks continue to grow. The unusual feature of zirconium oxide ceramic in comparison with other ceramics is that at the appearance of a high-tension area a transformation of the crystal structure can take place. This process is also accompanied by a volume expansion. By this volume increase it builds wedges in the crack and therefore it reduces the continuation of the crack. While the critical tensile strength (KIC) e.g. ofDicor, Vita Mark II and Empress is in the area of 1-2.5 Mpam-1/2, zirconium oxide shows values in the range of 10 Mpam-1/2. Even In-Ceram (glass infiltrated Al203 ceramic) and Procera aluminum oxide (pure Al203 ceramic) show values less then 5 Mpam-1/2.
In connection with the tensile strength there also stands the characteristic of bending strengths. While conventional glass ceramics show results of 100-200 Mpa and aluminum oxide ceramics lie in the area of 400-600 Mpa, zirconium oxide reaches a bending strength of over 1000 Mpa.
Because of the high tensile strengths exhibited in test results, it is now possible to fabricate posterior bridges with zirconium oxide. Further decisive advantages of zirconium oxide are its high resistance to corrosion; stability to hydrolysis and its high biocompatibility in comparison with other ceramics makes this material ideal for restorative dentistry.
In medicine, zirconium oxide is being used more and more as the material of choice especially for hip prosthesis. For years there has existed substantial clinical tests and examinations which confirm the high quality of zirconium oxide.
All Ceramic Restorations
new Procera® Crown Zirconia 0.4
The ever-popular Procera® Crown Zirconia is now even thinner yet still has exceptional strength
- Thinner coping enables more flexible esthetic opportunities
- Combines zirconia extreme flexural strength, 1200 MPa, with a beautiful esthetic result
Procera® Crown Zirconia
- Fracture rate less than 0.5%
- Proven strength and all-ceramic beauty
- Highly biocompatible material
- No specialized clinical preparation or cementation procedures
- Zirconia for the highest load situations
Biocompatible Denture Materials
Denture Materials
Today dentists are prescribing flexible material for removable partial dentures (RPDs) because it makes a better, stronger appliance faster. Flexible material reduces chair time, eliminates invasive procedures and the cumbersome materials associated with rigid partials. In short, there is no longer any need for metal.
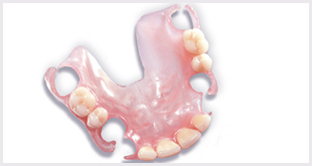
Metal-based RPD design is complex because it has to adapt rigid materials to a flexible environment. This leaves room for error particularly under conditions where ideal designs and clinical preparations are challenged.
In contrast, the material in flexible partials is perfectly suited to the variety of natural conditions in the mouth. It simplifies the design and enables the RPD itself to balance the simultaneous requirements of retention, support and stability.
Flexibility removes many of the unwanted aspects of metal partials. There are fewer steps in the treatment process because preparation of natural teeth is unnecessary. Without the metal frame, the fabrication and try-in processes have also been simplified. In addition, the choice of a Valplast® or Lucitone FRS partial avoids the placement of metal in your patient’s mouth, which allows you to satisfy the patient’s interest in metal-free dentistry.
When you prescribe a genuine Valplast or Lucitone FRS partial, you give your patient far more than just a dental appliance. Valplast or Lucitone FRS takes a comprehensive approach to both the science and the business of flexible partials. Since the beginning, it has been our mission to elevate removable partial dentures to a higher-level of function, simplicity and patient satisfaction. We provide the industry’s most proven flexible RPD material backed by the most extensive knowledge, experience, training and advanced equipment.
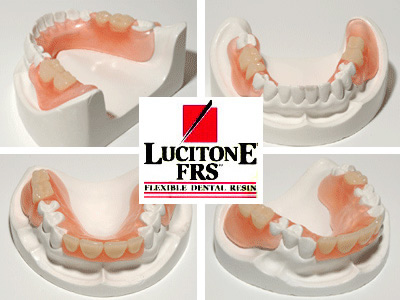

Valplast Partials

Valplast is a flexible denture base resin that has been used in dentistry since 1954 and is ideal for partial dentures and unilateral restorations. The resin is a biocompatible nylon thermoplastic with unique physical and aesthetic properties that provides unlimited design versatility and eliminates the concern about acrylic allergies. It allows your patient’s natural tissue tone to appear through the material, matching the basic shade categories (medium, light pink, and meharry). Patient’s report that it feels more comfortable because of the thinness and light weight. It is also guaranteed for life against breakage when fabricated and handled according to the manufacturer’s specified procedures.
Valplast Nesbit and Flipper

The Valplast Nesbit is ideal for posterior single tooth replacement and the Valplast Flipper is ideal for anterior single tooth replacement. The design of the new flexible plastic framework takes the danger out of an accidental swallowing of the appliance. In the event that someone did swallow one, it is unlikely that any damage could be done to the lining of the digestive system.
Bonding
Below is a white composite filling. This is the most common filling material used to replace mercury fillings. A direct composite is made up of plastic resin, reinforced with filler particles of silicone dioxide (glass) or zirconium.

Since all direct fillings (composites) and most indirect restorations (inlays, onlays, and crowns) being placed today use a process called bonding, it’s good to have a basic knowledge of how this process works. While there are a myriad of variations in this process, here are the basic steps:
Step 1–Prepare the tooth surface using a mild acid solution. This creates a “honeycomb” in the top layer of tooth.
Step 2–Paint a liquid resin-bonding agent on the tooth. It flows and “locks” into the honeycomb created in Step 1 (technically forming a hybrid layer that is part-tooth and part dental-resin). This layer is “cured” (hardened using a photo-chemical reaction) with a visible light source.
Step 3–Place luting cement if an indirect restoration (e.g., crown) is being used. Essentially, this material is a more liquid form of white filling material. It bonds to both the tooth and the pre-fabricated ceramic restoration and fills the gaps between them. The surface of the first layer of cured bonding agent is highly reactive and is easily bonded to with today’s composite filling materials.
Advantages of Bonding
There are a number of advantages to using bonding. Besides allowing the placement of non-metal restorations, less removal of healthy tooth structure is required. The dentist needs to remove only the unhealthy tooth structure and a precisely fitted piece can be bonded into place to replace it. In the case of fillings, bonded restorations have other physical advantages over amalgam. Even the ADA, in its Journal of the American Dental Association (JADA) , recently acknowledged, “Resin-based composites eventually will replace amalgam as a direct restorative material because they possess many characteristics not inherent in amalgam. Some of the more important of these properties are esthetics, micro-mechanical bonding to tooth structure, smaller cavity preparations and better sealing potential.” (JADA, Vol. 132, August 2001, p. 1100).
New Advances
Breakthroughs in the development of dental materials and techniques are occurring on a regular basis. We have yet to find one single material that is compatible for everyone. Debate continues about how to best restore a patient’s mouth to optimal health and no perfect solution has yet presented itself. The best we can do is to to keep an open mind and keep searching for answers. Only by thorough testing can it be determined which is most compatible for any one person.
Testing for Dental Compatible Material
All dental materials should be tested for compatibility prior to their use in order to insure maximum safety and good health. You cannot assume that your body will handle all material for you. It is important to include dental material testing in your decision making process.
Blood Compatible Reactivity Testing
Using serum from a patient’s blood sample, antibodies formed against the chemical groups that result from dental product breakdown are detectable . Once the patient is sensitized from any source and is producing antibodies, use of any product that contains or may give off these substances should be avoided. This procedure makes it possible to select those materials that are least likely to cause a reactivity problem. This special testing service which can help to reduce you risk from adverse reactions with dental materials. This procedure can be performed from a simple blood specimen. Also provided that it is used as part of a risk assessment program to assist the doctor in selecting those materials which may be safest to use with the patient.

Biocompatibility/Clifford Testing
If you are concerned about the biocompatibility of the materials used to restore your teeth, we recommend the Clifford Materials Reactivity Test. This test only requires a blood sample, and from that little sample, it can be determined, among the myriad of materials available, which ones are most suitable for you!
Clifford Materials Reactivity Testing (CMRT) is a laboratory screening process used to help identify existing sensitivity problems to various chemical groups and families of compounds in an individual patient. This process is currently being implemented in the CMRT Dental Test. After a patient’s test has been completed, the patient’s reactivity test results are compiled in a report. The test currently reports on over 7100 trade-named products and 89 chemical groups and families.
The Clifford Materials Reactivity Testing can be useful for any patient who is preparing for restorative or reconstructive bio-materials placement and who would like to have some additional peace of mind that the materials proposed for use are not likely to induce adverse bio-reactivity. Additionally, there are several groups of patients who might derive special benefits from the added safety of materials reactivity pre-assessment testing.
Some of these groups include:
- Any patient who has experienced difficulty with prior materials placement
- Patients with severe immune-compromised status
- Patients with identified autoimmune diseases or conditions
- Endocrine, hepatic or renal compromised patients
- Patients with universal reactor/environmental illness status
- Patients with severe allergic problems
- Patiens with known sensitivity to jewelry, watchbands, clothing fasteners
- Patients with identified galvanic or bruxing issues
- Patients who receive daily does of numerous medications
- Patients facing high-cost, complex restoration projects
- Patients previously treated by multiple unrelated healthcare professionals
Read more at: biodentistrydrvizcarra.com
Tagged Under: Biocompatible, Holistic Dentistry